
Japan Market Access Forum (J-MAF) Updates
2025.5.30
Foreign Reference Pricing for New Devices at Initial Listing
President Trump’s Executive Order earlier this month that established Most-Favored Nation (MFN) pricing for the Centers for Medicare and Medicaid Services (CMS), the world’s largest single purchaser of prescription drugs, sparked a lot of discussion among the market access specialists and pharma industry professionals in my network. The question is how drug manufacturers will adjust their planning and launch strategies around the world, given that the US will be referencing the lowest prices from a basket of other developed countries. It will be interesting to see how things play out. Japan references the US, UK, Germany, and France for new drug listings and will make an upward or downward adjustment if the price initially calculated for Japan, based on the calculation rules we use, is less than 75% or more than 125% of the average foreign reference price.
Although medical devices may not be affected by the new Executive Order, foreign reference pricing for medical devices in Japan has been common practice for over two decades. Japan currently references market prices in the US, UK, Germany, France, and Australia and considers the average price for those markets. While foreign reference pricing only happens once in Japan for prescription drugs, for medical devices it is considered both at initial listing and when the actual market price exceeds the average foreign reference price by 1.3 times. There is also a rule for medical device reimbursement in Japan that says that the initial reimbursement price in Japan cannot exceed 1.25 times the average foreign reference price. However, for certain novel devices, those designated as high medical needs devices, or those that receive orphan device designation or Specific use designation, the upper limit of the reimbursement price requested for Japan is 1.5 times the average foreign reference price.
We did a quick review of the ratio of the average foreign reference prices mentioned in listing reports for newly reimbursement prices to the price initially calculated for Japan for unique devices newly reimbursed between fiscal year (FY) 2015 and FY 2024 in Japan. To avoid double counting, we excluded some device components that were clearly part of the same device set and had the same price / price ratio for each component in the set. Also, devices that did not report a reference price for any of the reference markets were excluded – which accounted for about one-third of newly reimbursed devices.
In total we reviewed 137 devices newly listed between FY 2015 and FY 2024. In Figure 1 below, you can see that for nearly 70% of devices, the initial reimbursement price for Japan was less than 100% of the average foreign reference price meaning that the initial price for Japan is typically less than average foreign reference price. However, just looking at the 73 devices that were newly reimbursed between FY 2020 and FY 2024, only about 58% were less than 100% of the average foreign reference price compared to around 80% for those newly reimbursed between FY 2015 and FY 2019. So, while the situation seems to have improved in recent years, the initial reimbursement price is still typically less than the average foreign reference price for newly listed devices in Japan.
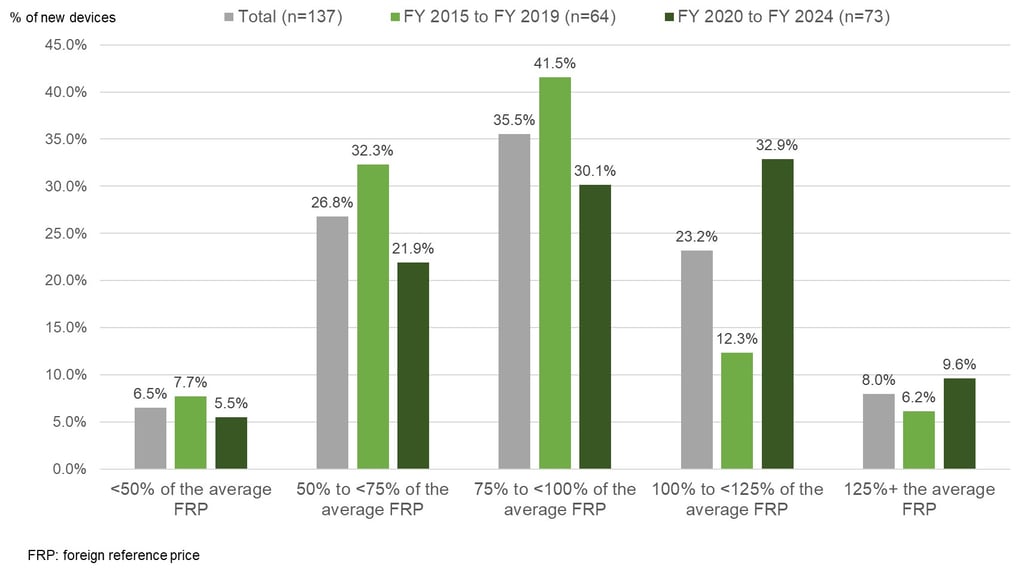
Figure 1: Percentage of Newly Reimbursed Devices By The Ratio of their Initial Price in Japan
Relative to the Average Foreign Reference Price
In Figure 2 below you can see how the situation differs by therapy area. The sample sizes are small, but the initial reimbursement price for devices treating cardiovascular conditions in Japan is more often below the average foreign reference price at listing. This is interesting given that a recent study showed that devices treating cardiovascular conditions are more likely to receive a reimbursement premium (see here). Again, while the sample size is small, wound care products seem to have done well, with around 77% of those products receiving an initial reimbursement price in Japan that was at least the same as – if not more than – the average foreign reference price.
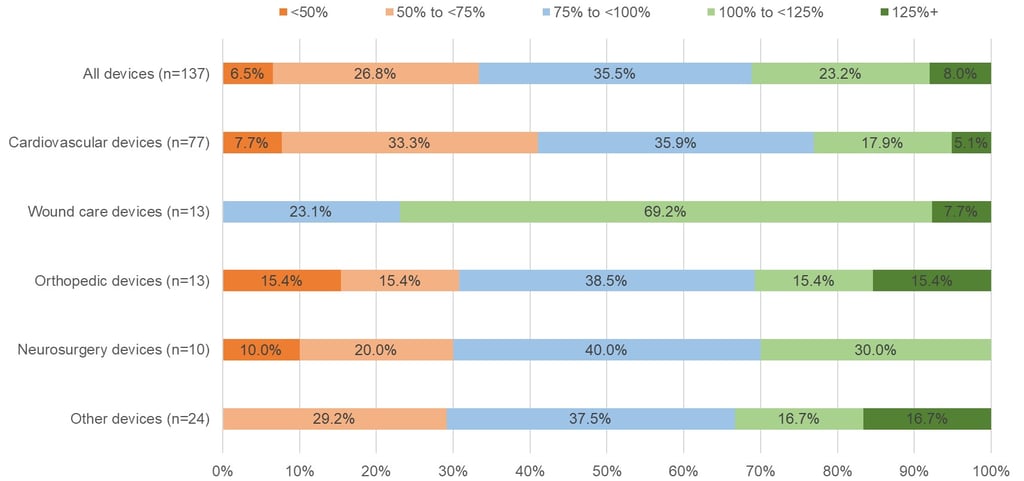
Figure 2: Percentage of Newly Reimbursed Devices By The Ratio of their Initial Price in Japan
Relative to the Average Foreign Reference Price, By Therapy Area
Lastly, Figure 3 below shows what the ratio is typically like for the price in each of the reference markets versus the initial price calculated for Japan. Overall, the initial price calculated for Japan is about 85% of the average foreign reference price, on average, but there were large differences by country. The initial reimbursement price for Japan was, on average, about 68% of the US market price, 85% of the UK market price, 89% of the Germany market price, and 99% of the Australia market price. However, the initial reimbursement price for Japan price was often higher than the market price in France at 102%, on average.
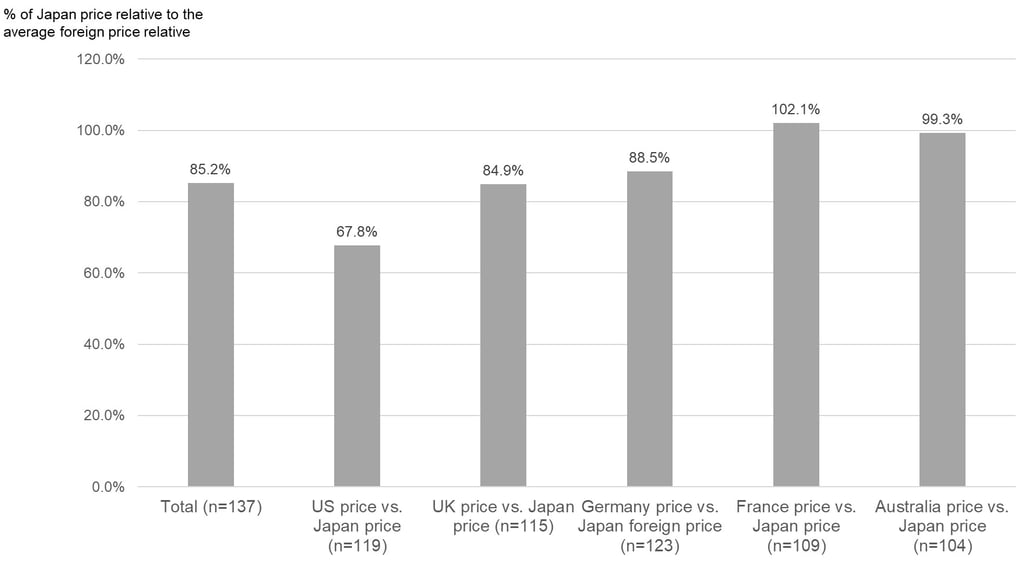
Figure 3: Average Percentage (Ratio) of Initial Price for Newly Reimbursed Devices in Japan
Relative to the Price in Each of the Reference Markets
As shown in our FY 2024 new device listing here, the ratio of the initial reimbursement price for Japan to the average foreign reference price for devices newly reimbursed in FY 2024 varied significantly by device. However, the above analysis may give you an idea about the general framework for initial reimbursement prices for Japan relative to market prices in the reference markets – when one or more reference prices are available. However, you should keep in mind that foreign reference pricing doesn’t just happen at listing for devices in Japan and there is a mechanism that will adjust a new reimbursement price down by up to 50% if it exceeds the average foreign reference price by more than 1.3 times after listing. That rule, however, currently does not apply for devices (functional categories) that cover only children and rare diseases, and functional categories for which prices were revised because they were determined to be extremely difficult to supply.
Perhaps in the future we will look at how post-listing foreign reference price adjustments have affected reimbursement pricing in Japan, but we suspect that competition due to other competitor devices being listed in Japan may be a bigger driver of price reductions for devices in Japan. Nonetheless, significant reductions in device reimbursement prices outside Japan may impact prices in Japan.
For a more detailed overview or support with device reimbursement strategies in Japan, please contact us.
Subscribe to receive Japan MedTech Access Forum (J-MAF) updates
We respect your privacy. You will NOT be sent relentless emails by GinePro!


Healthcare evidence generation & access support for Japan.
© 2024-2025 GinePro LLC. All rights reserved.


