
Japan Market Access Forum (J-MAF) Updates
2024.12.23
Examples of the New Economic Efficiency Premium
In April 2024, a new reimbursement premium was introduced in Japan for reimbursed medical devices, the Economic Efficiency premium. While there are several premiums available for newly reimbursed devices in Japan which are described in detail here, this is a reimbursement premium unlike any that we have seen before in Japan. This premium allows for part of the cost savings from the use of a new device to be incorporated directly into the reimbursement price for the new device. This premium is applied when a new medical device (1) targets essentially the same condition and has the same intended use as an already listed device, (2) it demonstrates clinical efficacy that is equal to or better than the already listed device, (3) it can serve as a substitute for the already listed device, and (4) it is expected to reduce costs related to insurance medical devices compared to using the already listed device. With this premium, 50% of the anticipated cost savings from eliminating the use of other devices is awarded as a premium for the per unit reimbursement price of the new medical device.
This new premium was introduced based on recommendations from the device industry. The basic principle if that even if a new device is more expensive than existing devices, it may lead to a reduction in costs overall if it allows for the elimination of use of other devices. The example given in the reimbursement revision report is the case whereby, while conventional deep brain stimulation (DBS) therapy for Parkinson’s disease might require two devices with two leads (e.g., Medtronic Activa PC/S/RC), a new device might allow for just one device with two leads (e.g., Medtronic Percept PC). With the new premium, 50% of costs savings from eliminating the need for other devices may be incorporated into the reimbursement of the new device.
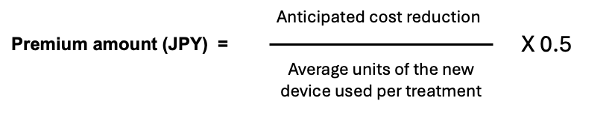
The premium amount itself is calculated by dividing the anticipated cost reduction with the new device by the anticipated average number of units of the device used per treatment and multiplying that amount by 0.5 as show below:
The cost reduction is “said to be” calculated by subtracting the product of the average number of units of the new device and the reimbursement price of the already listed device from the product of the average number of units of the already listed device and reimbursement price of the already listed device. This sounds rather like a very basic budget impact analysis, which all devices must include in their dossier submission. But, looking at a recent example of its application, the calculation for the premium seems a bit more straight-forward and is more in line with the intention of the premium as described in the 2nd paragraph above.
Since April 2024, we have seen this premium awarded for two newly listed devices: Johnson & Johnson’s VARIPULSE Pulsed Field Ablation Catheter and Medtronic’s PulseSelect PFA Loop Catheter. Both were listed in September 2024 and a detailed overview of their reimbursement decision can be found here. For both devices the projected cost savings was determined by using the “114 Catheter electrode for extracorporeal pacemakers: (2) Cardiac electrophysiology test function added type 2) Coronary sinus type" functional category which is 64,000 JPY per unit. As such, the premium allowed was 32,000 JPY per unit (64,000 JPY x 0.5 = 32,000 JPY). So, the notion seems to be that when using the VARIPULSE Pulsed Field Ablation Catheter or the PulseSelect PFA loop, the use of a coronary sinus-type catheter electrode for extracorporeal pacemakers that has cardiac electrophysiology test functionality (e.g., Johnson & Johnson’s Lasso NAV 20mm, etc.) can be eliminated.
It is hoped this new premium will help mitigate any disincentives for manufacturers to create more economic efficient versions of existing technologies, whereby launches those products might mean that products that they already sell may be used less or become obsolete. Since it can be challenging for medical devices to prepare cost-effectiveness analysis prior to listing due to lack of data incentives that reward technological innovation like this new premium are very encouraging and will help to improve access to new technologies for patients in Japan.
Closing thoughts:
Our feeling is that the Economic Efficiency premium is a big step for medical device reimbursement in Japan – which is an area where more incentives for introducing new technologies are really needed. It is not uncommon for new premiums (or rationale for premiums) to be established for medical devices without much practical usage or application. (Please see our publication here for more information on that problem.) So, it is encouraging that we have already seen some examples of this premium being applied. However, manufacturers must be prepared to present evidence on how their new device will affect the use of other devices when reimbursement is considered. As mentioned above, a basic budget impact analysis is required for all new devices (and procedures) in Japan. So, a simple supplemental analysis that shows how the use of other devices may be reduced could be done in addition to that analysis. GinePro has expertise with conducting budget impact analyses for new medical devices in Japan and we can support with an assessment of a potential Economic Efficiency premium as part of that process. Please contact GinePro if you would like to hear more about those services.
Subscribe to receive Japan MedTech Access Forum (J-MAF) updates
We respect your privacy. You will NOT be sent relentless emails by GinePro!


Healthcare evidence generation & access support for Japan.
© 2024-2025 GinePro LLC. All rights reserved.


